Here is a simplified description of the life cycle of lichens, which can involve vegetative or asexual reproduction as well as sexual reproduction. The features of the three main reproductive strategies are summarised in Table 1, showing how the mycobiont (fungus) and photobiont (e.g. algae) re-combine in offspring.
Table 1 Three Reproduction Strategies in Lichens
| Means of reproduction | Mechanism | Genetic consequences | Re-combination with photobiont | Final result |
| Vegetative Reproduction | Thallus fragments break off, e.g. soredia or isidia containing both mycobiont and photobiont. These fragments form a new thallus in a suitable location. | Offspring of both mycobiont and photobiont genetically identical i.e. a clone. | No new combination of mycobiont and photobiont | Same combination of photobiont and mycobiont |
| Sexual Reproduction | Mycobiont pycnidia eject microconidia that join with ascogonia on another thallus. These develop into ascomata fruiting bodies that eject ascospores that combine with new photobiont to form a new thallus. | Gene exchange and recombination in offspring. Usually two genetically different individuals participate, although some species can self-fertilise | New combination with photobiont | Both photobiont and mycobiont possibly genetically different from original thallus |
| Asexual Reproduction in Mycobiont | Mycobiont pycnidia eject macroconidia that combine with new photobiont to form a new thallus. | Mycobiont genetically identical i.e. a clone of mycobiont | New combination with photobiont | Mycobiont unchanged but new photobiont selected can be genetically different |
Vegetative reproduction
All lichens can reproduce vegetatively. Any small bit of the vegetative part of the thallus containing the two symbionts can potentially grow into a new individual. In this way, lichens, like some other organisms such as mosses, are called totipotent, i.e. any small piece can make a new individual. Fragments can move in or on the surface of water films during rainfall and mites and slugs etc may also move thallus fragments, either on their bodies or through their guts and in their droppings. Thallus fragmentation is exploited by some lichens in the production of soredia, isidia, or other features that aid the breaking off of bits of thallus. This could include the brittleness in Cladonia when the thalli are dry.
Small fragments (e.g. soredia or broken-off isidia) adhere to the substrate and immediately start to form a stratified thallus. In foliose lichens, a lobe emerges, branches and develops a more or less circular thallus. For both foliose and crustose species, the circular thallus forms a growing edge and a mature central portion. This central part can then form more soredia or isidia and so start the process again. Fruticose species form a holdfast and a vertical branch from the original fragment.
This means of reproduction is more certain and can be quicker than producing spores. For example, in common species such as Parmelia sulcata, Physcia tenella or Lecanora orosthea, soredia can give rise to a population of numerous identical thalli in quite a small area. Although most lichen species in the world only reproduce sexually by the mycobiont producing spores, vegetative reproduction is a feature of most common and abundant lichens because they can colonise and spread quickly. Interestingly, just because a lichen has soredia (or other vegetative reproductive organs) it does not mean that these species do not reproduce sexually as well – mostly they do but may be not as often as other non-vegetatively reproducing lichens.
Vegetative reproduction does have limitations (Table 1). Genetic variation arises slowly by random mutation especially if gene segregation and recombination (which are features of meiosis) do not happen. With the Darwinian process of evolution by natural selection, adaptation to a changing or new environment may be slower and less effective with vegetative reproduction than would be the case for sexually reproducing species.
The terms used for vegetative propagules are often confusing. Table 2 indicates some of the characteristics of the better-known propagule types. Some isidia break down into soredia and vice versa with soredia turning into isidia-like propagules as with isidiomorphs. In the common Peltigera, P. praetextata, the isidia like structures are called schizidia in LBGI probably because they do not have a lower cortex. (Peltigeras do never have a lower cortex.) But actually they are better describes as phyllidia by the way they are formed.
Table 2 Types of vegetative propagules in lichens
| Type of vegetative propagule (singular in brackets) | Characteristics | Where formed | How common. With examples of species |
Soredia (Soredium) | Minute balls of mycobiont and photobiont. Unwettable due to a covering of hydrophobins | Over the surface of the thallus or just in soralia (singular soralium) Usually on upper surface but on under surface in some species. | Very common. Physcia tenella, Parmelia sulcata |
Isidia (Isidium) | Tiny vertical outgrowths. Finger-like or branched, like coral, or spherical. With cortex, easily broken off. Wettable | Usually on the upper surface of the thallus | Very common. Parmelia saxatilis, Lepra corallina |
Blastidia (Blastidium) | Horizontal outgrowths with cortex rather like isidia. Often in chains | On the edge of thallus lobes or areoles | Frequent Caloplaca arcis, Lecania erysibe |
Phyllidia (Phyllidium) | Lobe-like outgrowths which can break off | Usually on edges of lobes | Not very common. Peltigera praetextata |
Schizidia (Schizidium) | Flaking of the surface layers of the thallus including cortex and photobiont layer | On surface of thallus | Rare Baeomyces or Dibaeis spp |
Goniocysts (Goniocyst) | Goniocysts are formed around a single photobiont cell which then divides. A soredium is formed by the grouping existing photobiont cells. See Serusiaux E (1985) Lichenologist 17 1-25 | In goniocystangia (goniocystangium) | Some species of Agonimia, Bacidia, Micarea - and Normandina acroglypta |
Hormocysts (Hormocyst) | Cyanobacterial chain (hormogonia) wrapped in hyphae and formed in a similar way to goniocysts | In hormocystangia (hormocystangium) | Rare. In cyanobacterial lichens. Lempholemma spp. |
Mycobiont Sexual Reproduction
Some lichens reproduce by sexual reproduction of the mycobiont (fungus), particularly if they do not produce isidia or soredia etc. Some lichens use both sexual and vegetative reproduction.
In sexual reproduction, lichens create ‘fruiting bodies’ called ascomata (singular: ascoma). These ascomata release microscopic mycobiont spores over a long period (months to years). Thousands of the spores are dispersed by wind, rain and animals. If a spore arrives at a site where substrate and location are suited to the lichen, and it finds a photobiont (i.e. alga or cyanobacterium) cell nearby, a new lichen thallus can develop. First, the spore germinates and sends out a thread (hypha) which grows and branches forming a microscopic network of hyphae called a spore mat (Figure 1). One of these hyphae, touching an algal cell, will envelop it and attempt to take nutrition from it. If the algal cell is a potential photobiont, the hyphae will allow it to divide. At the same time, the hyphae will absorb sugars realised by the photobiont cell. A cluster of several photobiont cells with the mycobiont hyphae is effectively an embryonic lichen thallus (termed a Minimum Unit of Lichen Development by the American lichenologist Vernon Ahmadjian) from which a normal thallus structure will grow. (Had the hyphae from the spore enveloped an algal cell that could not be a photobiont, the algal cell will die. The mycobiont may obtain some intermittent nutrition from non-symbiotic interactions with unacceptable algal cells while seeking a photobiont.) The attachment of the hypha to the initial photobiont cell may involve lectins: proteins that bind onto carbohydrates such as those that make up the photobiont cell wall. The presence of the lectins has been identified as part of a mechanism for the identification or selection of suitable photobiont strains by the Israeli lichenologist Margalith Galun.
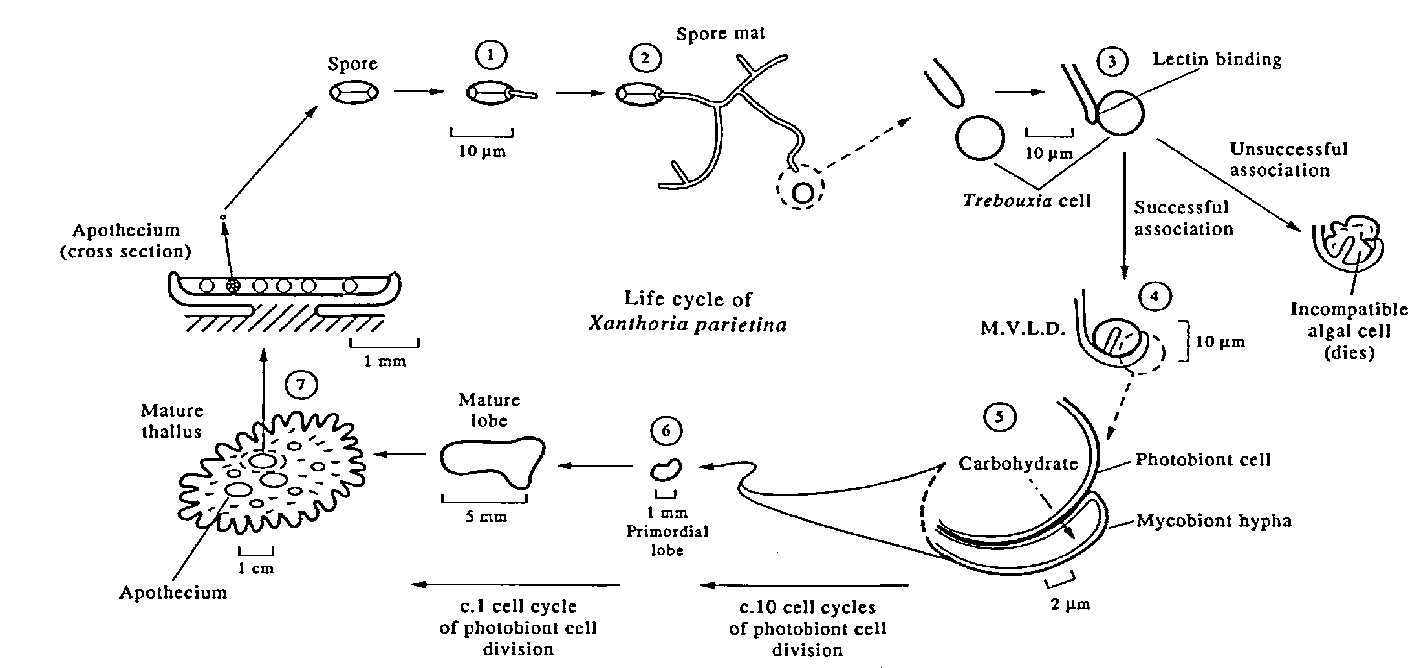
Figure 1 Life cycle of Xanthoria parietina. (From: Hill DJ (1994) Endeavour, New Series 18(3) 96-103)
The Details of Mycobiont Sexual Reproduction
Only the mycobiont appears to reproduce sexually in lichens. Fungi that belong to the Ascomycota phylum reproduce sexually by producing spores in a sac called an ascus (plural asci). In Basidiomycota spores are formed outside a cell (called a basidium) at the ends of horns. Although most lichen mycobionts belong to the Ascomycota, a few mycobionts belong to the Basidiomycota phylum.
Asci are produced in special fruiting bodies called ascomata. They can be referred to as ascocarps or colloquially as fruiting bodies. There are two main types of ascomata as follows:
Apothecia
Apothecia (singular apothecium) are disc or saucer shaped and usually have a rim round them (Figure 2). The asci are lined up with vertical supporting hairs (called paraphyses singular paraphysis) in a central layer and fire their spores vertically into the air. Some are compacted side-ways, so the rims meet to form a long thin ascocarp with the two rims side by side and the asci in between. These are also termed lirellae (singular lirella) (Figures 4 and 5).

Figure 2 Ricasolia virens (formally Lobaria virens) with brown apothecia

Figure 3 Section through an apothecium of Amandinea punctata. Spores in the asci are brown and between the asci are numerous paraphyses. (Photo Juliet Bailey)

Figure 4 Graphis scripta with black lirellae (Photo Neil Sanderson)
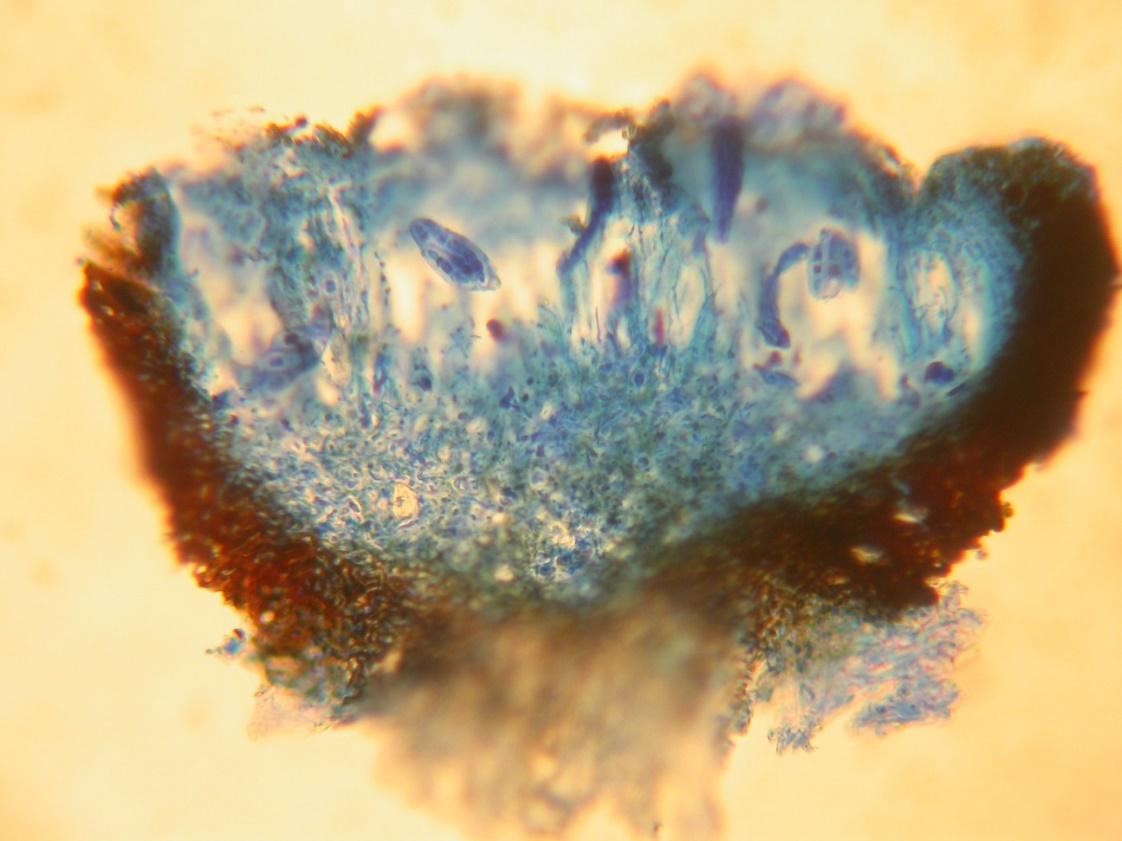
Figure 5 Section through a lirella (Alyxoria varia)
Perithecia
Perithecia (singular perithecium) are flask shaped with a central pore (ostiole) in the top through which the spores are fired (Figures 6 and 7). They have vertical supporting hairs and downward pointing periphyses (singular periphysis) round the inside of the pore.

Figure 6 Parabagliettoa dufourii with black perithecia each with a pore in the top out of which spores are fired.
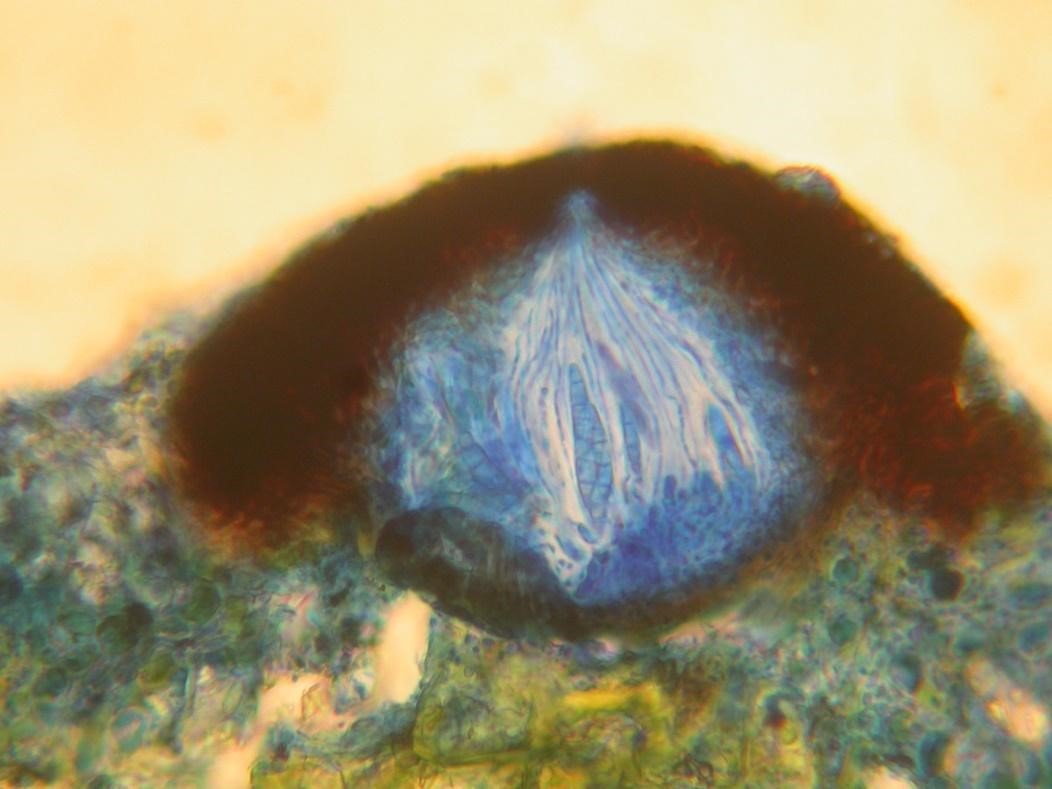
Figure 7 Section through a perithecium of Porina byssacea
Mazaedia
A few species of lichens produce apothecia on a stalk (Figure 8) and the spores are released as a loose mass (mazaedium). Also known as pin-head lichens, or coniocarps, these are usually found in cracks on the dry side of tree trunks.

Figure 8 Calicium viride with apothecia forming a mazaedium on stalks
Formation of ascomata
Sexual reproduction requires two separate genetically different individuals. In fungi these individuals are not categorised as male or female. Rather than having genders, fungi have mating types. As long as the two individuals belong to different and compatible mating types, they can participate in the formation of ascomata. There can be two or more mating types in a population of sexually reproducing fungi but not all pairs of them may be compatible.
Some of the stages in the formation of ascomata in the ascomycota (Figure 9), often referred to as ascocarp ontogeny, are quite difficult to observe. The ascocarp ontogeny of lichen mycobionts is quite diverse and taxonomically important. The following description is very simplified for the general account here and based partly on what is known about non-lichenised fungi. There is no reason to suppose that because a fungus is lichenised, the sexual process will be different.
Typically, an individual mycobiont will produce pycnidia (singular pycnidium) which are flask-shaped and exude thousands of (macro) conidia (singular: conidium). Conidia are asexual spores about the same size as ascospores that function in asexual reproduction of the mycobiont. Pycnidia can also produce very small conidia often referred to as spermatia (singular spermatium) which act as gametes carrying nuclei to another thallus as sperm do in plants and animals. In identification books spermatia are usually called microconidia. Spermatia may be only a few microns long and one or two microns wide (about the same size as a bacterial cell) and so small they can only be observed with powerful microscopes that magnify 1000 times. Because they are so small, spermatia only seem to function as a carrier of a nucleus, there being no space in the spermatium for any food reserves that characterise spores capable of germinating to form a new thallus. Spermatia travel easily on water and droplets and are so small they do not easily settle out of the air or water. Because they are so small, they are very effective at making their way to another thallus.
In the meantime, another thallus may be forming ascogonia (singular ascogonium). An ascogonium is a coil or knot of hyphae inside the lichen thallus that will become the starting point of the development or formation of an ascoma. The ascogonium produces one or more vertically oriented hyphae that grow upwards and out of the surface of the thallus. Once outside, they may appear bent like the handle of a walking stick and are termed trichogynes. In the air, the trichogyne will capture a spermatium that has found its way to the top of a trichogyne. There, the touching cell walls of the adhering spermatium and the trichogyne are dissolved so that the nucleus in the spermatium can enter the trichogyne. The spermatium and trichogyne nuclei occupy the trichogyne but do not fuse. The two nuclei migrate down to the ascogonium where they form a number of binucleate hyphae (termed a dikaryon) at the base of the developing ascoma. At the same time, the ascogonium becomes an ascoma (e.g. an apothecium or perithecium). As the ascoma develops further, the cells with two nuclei form a side branch called a crozier which is a vertical elongated cell bent round like the letter n. At the top of this, a larger cell (containing the two nuclei) grows up to make an ascus. As the ascus develops the two nuclei fuse and immediately divide by meiosis to form 4 new nuclei each with a different genetic make-up. These 4 cells will divide again to make 8 and be the basis for the 8 spores so characteristic of the ascomycota. In some lichens, two or more of the nuclei will abort and the ascus will contain fewer than 8 spores. In some other lichens, division of the nuclei after meiosis continues and we get multi-spored asci with up to 100 or so spores.
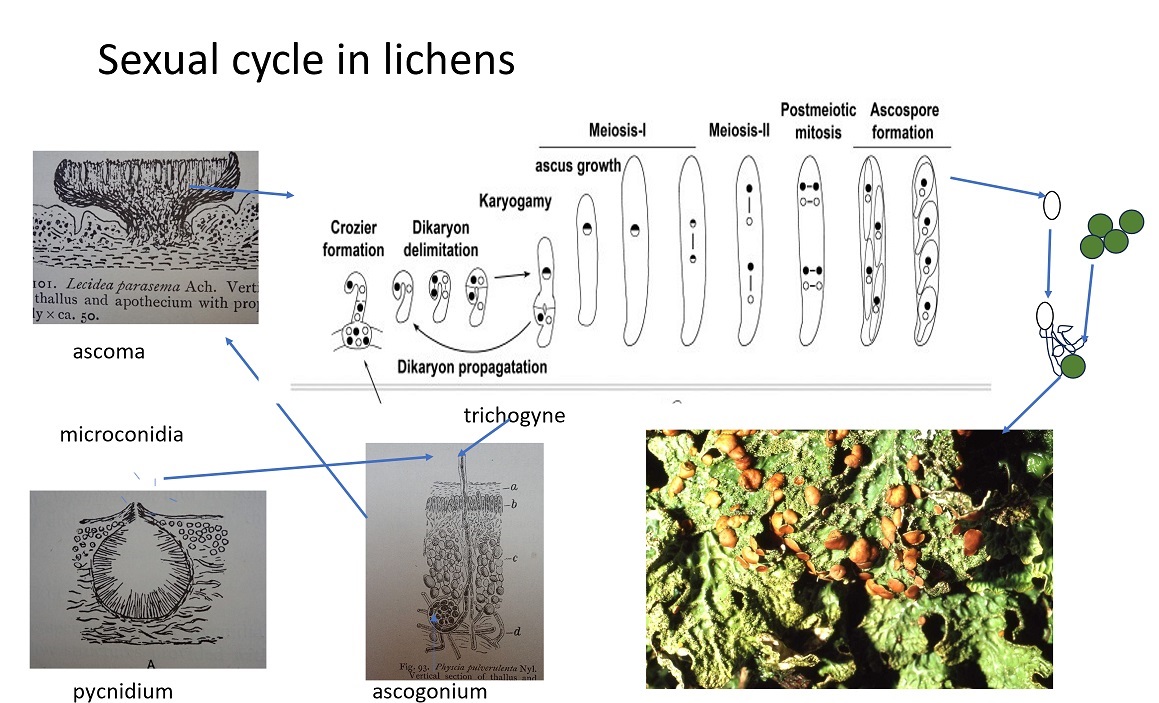
Figure 9 Sexual reproduction in mycobionts (various sources)
Ascus types and function
There are several different types of ascus. Recognising a type of ascus when examining a section of an ascocarp can be very helpful in lichen identification. There are two main types – unitunicate and bitunicate. Unitunicate have one main ascus wall. During ascospore discharge, the ascus wall stretches and the ascus extends above the surface of the disc (epithecium) (or through the ostiole of a perithecium) then the spores shoot out of the top into the air. There are various microscopic details to be seen at the apex of the ascus that in many cases may be associated with the spore release mechanism. The structures can be revealed by staining with Iodine using Lugol’s reagent after treatment with K. Some lichens e.g. Schaereria and Porina seem to lack any visible mechanism. In bitunicate asci, there are two walls to the ascus. When spores are discharged the outer one opens at the top and the inner one extends above the epithecium (or through an ostiole) and when its apex opens, the spores fire off into the air. Asci differ in their shape too. In Arthonia the ascus is very broad and often sphaeroid. In Acrocordia the ascus is cylindrical with parallel sides. There are many other shapes in between these two.
Asci fire off the spores with some force. The spores may reach several millimetres above the ascocarp. Spores can be captured by suspending a microscope slide immediately above the ascocarp and the spores, being sticky, adhere to the surface. The force used to fire the spores is generated by osmotic pressure. Prior to discharge, the ascus contains a significant amount of glycogen. When mature, the ascus releases amylase which catalyses the hydrolysis of the glycogen to glucose. The massive increase in the number of molecules in solution in the fluid surrounding the spores causes the ascus to rapidly absorb water by osmosis from the surrounding tissues. Doing this in the limited space within the ascus causes a massive increase in hydrostatic pressure. This pressure is partly relieved by the ascus extending and then it dissipates altogether when the tip of the ascus opens and releases the spores with the surrounding fluid into the air. The spores do tend to stick together. When a group of spores travels through air as a single mass, they will travel much further than single spores. The dynamics of this process are interesting in many ways. Is it advantageous for spores to travel together as a lump or singly? Maybe Pertusaria species (that tend to have few huge spores or even just one) can send their spores further up into the air than other lichens. Considering their diversity, how spores function in travelling to their destination (i.e. to where they will successfully start a new lichen thallus) and how they function when they arrive in acquiring a photobiont is hard to investigate but nonetheless fascinating to think about.
Spore diversity
The main elements of diversity in spores are size, septation, shape, pigmentation and in some cases fragmentation. Septation of spores is the internal division of the spores into separate cells. In some books describing the same spore, we may read “spores one-septate” in others we might see “spores two celled”. The range of spore characters is quite large: size 2-400 microns (200x linear size or 40,000 times in volume); septation from single celled to 20-30 or more cells; shape from spherical to long and thin (2 x 60 microns); pigmentation colourless to dark brown due to melanin. In some lichens, colourless spores can become brownish when old or moribund. In Ascomycete fungi that have very long thin multi-septate spores such as in the insect pathogen Cordiceps, the spores fragment after discharge. Similar spores in lichens seem to remain intact. Some spores do fragment though, for example those released by Strigula taylorii.
Mating types and Heterothallism
Unlike plants and animals, which have just two sexes (with exceptions), fungi can have multiple genetically determined mating types. There can be two or more - up to a dozen or so. The different mating types do not have any expression of morphological difference. A thallus can produce both ascogonia and microconidia. Therefore, mating types cannot be considered equivalent to sexes. Male and female do not have any more meaning in fungi other than referring to microconidia as “male” (because they are small and move) and ascogonia as “female” (because they do not move). As in plants, though, a system of self-incompatibility exists in most lichens so that spermatia from a single mating type cannot fuse with the trichogyne of the same mating type thallus and form binucleate hyphae.
Fungi have a system of heterothallism to control which individual mates with another. In this system an individual is self-incompatible such that it cannot reproduce sexually on its own - its spermatia cannot fuse with its own trichogyne and cause the development of an ascoma. A genetically different individual is required. The required genetic difference is in specialist mating type genes. These genes exist in the population of individuals in different forms (alleles). The combination of two different alleles is needed for compatibility in sexual reproduction. Not all combinations of different alleles may yield compatibility. Species can have two mating types that are compatible or several possible mating type combinations.
You may notice that in some cases species that are normally fertile have no ascomata. For example, I have found Glaucomaria rupicola several times in a churchyard as a single thallus and without any apothecia. This could be explained by there being no other thalli of a compatible mating type present so the ascogonia were unable to develop.
Although many species are heterothallic requiring two genetically different individuals for successful sexual reproduction, other lichens are self-fertile and do not need another individual to produce apothecia. In Xanthoria parietina which are self-fertile or at least do not need another individual in the production of apothecia. In this species, the asci are produced by parthenogamy in which two identical nuclei in the crozier fuse with one another. Its relative Xanthoria calcicola is functionally heterothallic and needs two different thalli to be involved in producing apothecia. You will notice that X. parietina always has apothecia but X. calcicola only occasionally produces apothecia and when it does there are comparatively few on each thallus. Those lichens that infrequently produce ascoma may well be heterothallic. Although X. parietina is self-fertile, populations are genetically diverse. Populations of sexually reproducing species are usually genetically diverse. Just because a species is self-fertile, it does not mean crossing between genetically different individuals does not occur as well. In self-fertile lichens, the self-incompatibility mechanism is apparently not functional. In vegetative reproduction, in which new thalli growing together are identical to each other, populations can be genetically diverse across their geographical range.
Mycobiont asexual reproduction
As described above, lichens reproduce very successfully by the mycobiont producing spores sexually despite the added need to find a photobiont.
It is not surprising that many mycobionts also produce asexual spores that mimic the sexual ones. These asexual spores are termed conidia (singular conidium also termed macroconidia in books for identification). They are formed in pycnidia rather like the much smaller spermatia that transfer nuclei to another thallus in sexual reproduction. For some crustose lichens (such as Swinscowia calcarea and Eopyrenula grandicula) conidial reproduction appears to be the preferential means of forming new thalli.
Strigula taylorii example
Figure 10 shows the main structures for reproduction of Strigula using the three types of spore.
Sexual reproduction: pycnidia produce very small spermatia (microconidia). These are ejected and fertilise an ascogonium on another thallus to produce a perithecium. The perithecia contain asci, each with eight ascospores. These spores are initially simple but develop to have one septum when mature. The spores are ejected and encounter a photobiont to start a new thallus.
Asexual reproduction: larger pycnidia produce larger usually 1-septate macroconidia (conidiospores). These are ejected and encounter a photobiont to start a new thallus that is a clone of the mycobiont.
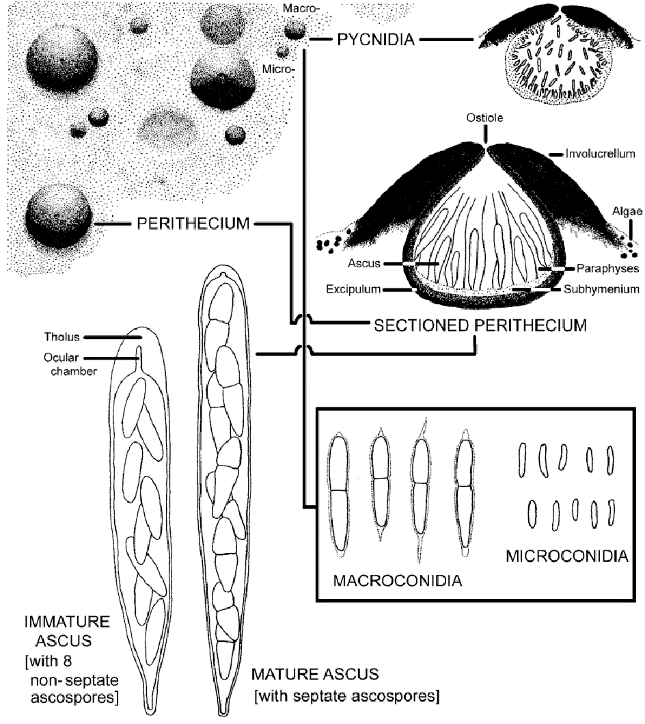
Figure 10 Spores and Ascoma of Strigula
Photobiont reproduction
Photobionts divide vegetatively in the lichen thallus. Evidence of sexual reproduction is very limited and generally has not been observed in lichen thalli.
The commonest algal photobiont Asterochloris reproduces sexually by forming motile gametes, a process that has been observed in liquid culture. It is quite possible that Asterochloris cells living outside the confines of a lichen thallus can produce motile gametes that can fuse to form new genotypes. Asterochloris populations have rarely been observed outside lichens.
Cyanobacteria do not undergo sexual reproduction. Genetic exchange in bacteria can occur, if rarely, by a process called conjugation. Conjugation in nature has not been observed in cyanobacteria but has been successful in cultures in the laboratory using bacterial vectors to deliver DNA sequences into the cyanobacterial cells. With the vast unlichenised populations of cyanobacteria such as Nostoc (which occurs in many lichen), some genetic exchange between individual strains at some time is almost certain.
Effectiveness of reproduction strategies
The extent of vegetative vs sexual reproduction in lichens is very difficult to assess. Unaided thallus fragmentation may be commoner than we may think – we just do not know. There are many possible vectors, including birds, snails, mites and molluscs.
Most vegetatively reproducing macro-lichens are common.
The proportion of species reproducing asexually varies from genus to genus eg:
- Xanthoparmelia in Australia out of 145 spp, only 4 are sorediate
- Usnea in Europe most species are sorediate
Most crustose species reproduce sexually.
In species that produce soredia or isidia, ascomata production is usually inhibited and when these species have ascomata, soredia or isidia production is usually inhibited. There are exceptions to this rule, for example, Fuscidea lightfootii and Pyrrhospora quernea.
Xanthoria parietina
Xanthoria parietina is self-fertile. It does not need another partner to make effective apothecia. Hence, it always has apothecia. Compare this with Xanthoria calcicola, which is often sterile.
Lobaria pulmonaria
Lobaria pulmonaria is heterothallic. This means there are two or more mating types and there needs to be one of each to make apothecia: one for making microconidia and one for making ascogonia and trichogynes which when fertilised leads to apothecia. Hence apothecia are usually only found within larger populations of this species.